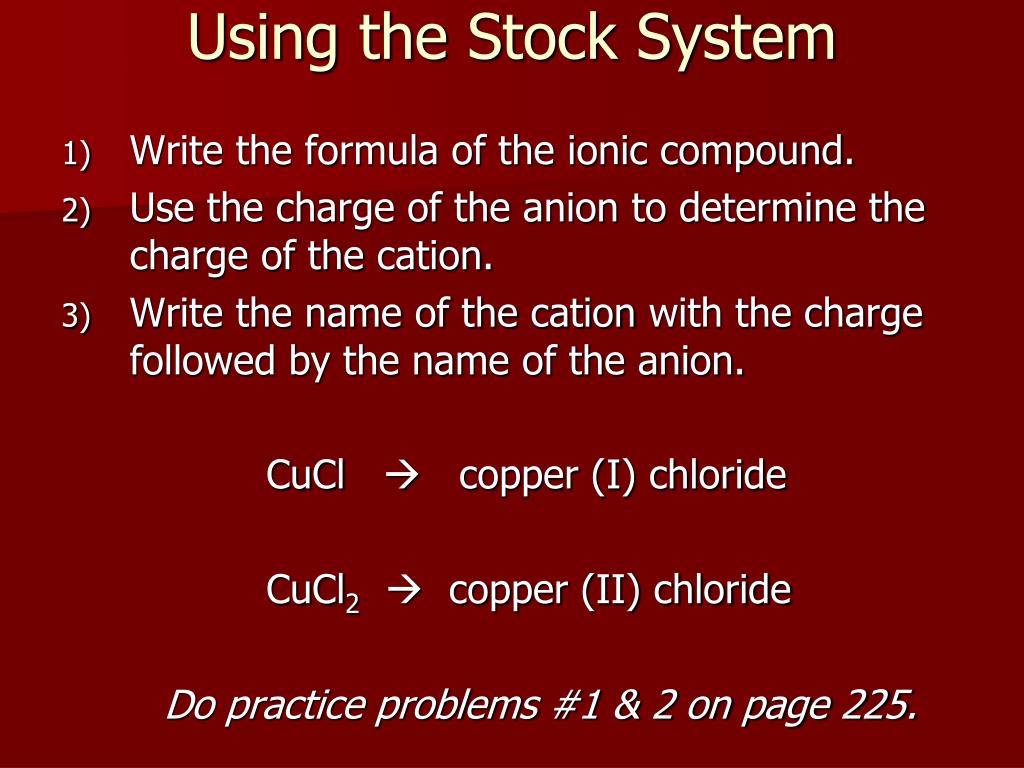
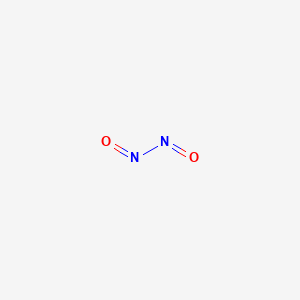
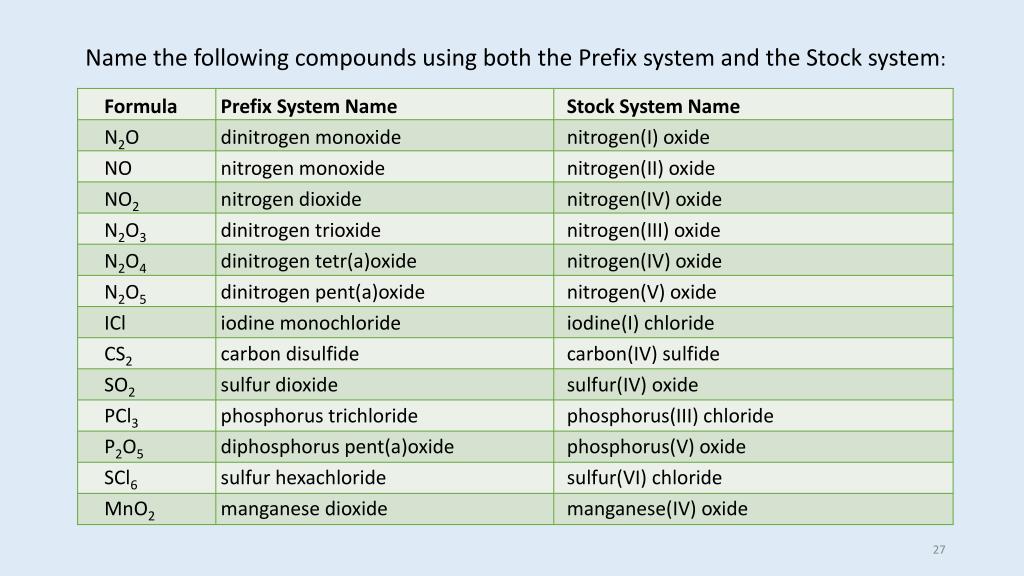
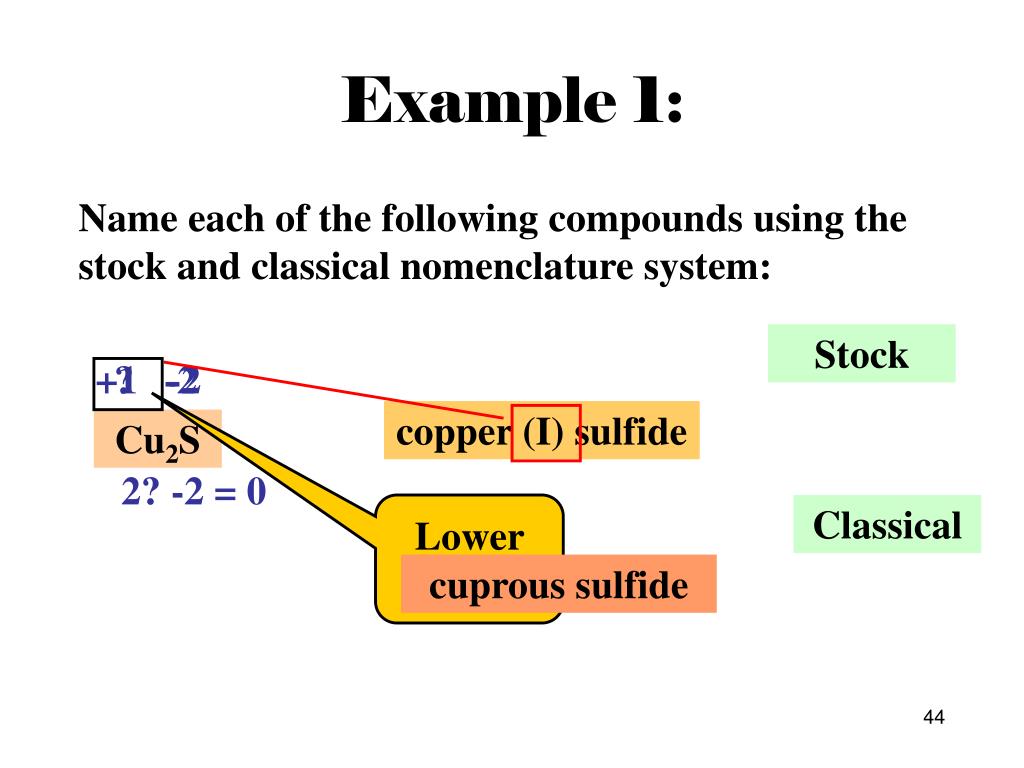
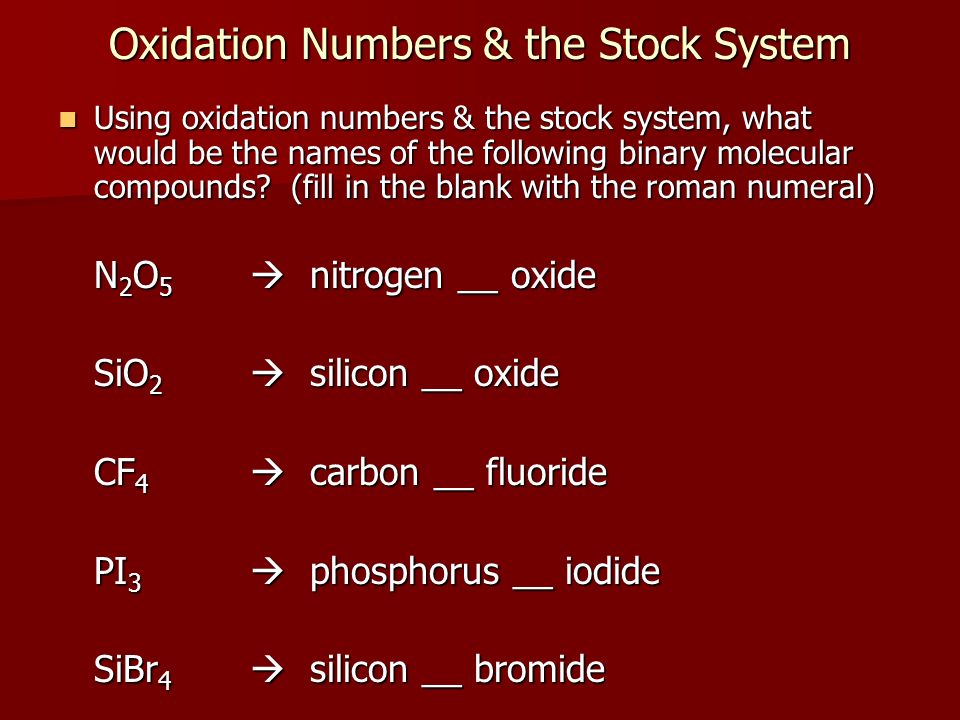
Name The Compound N2o2 Using The Stock System
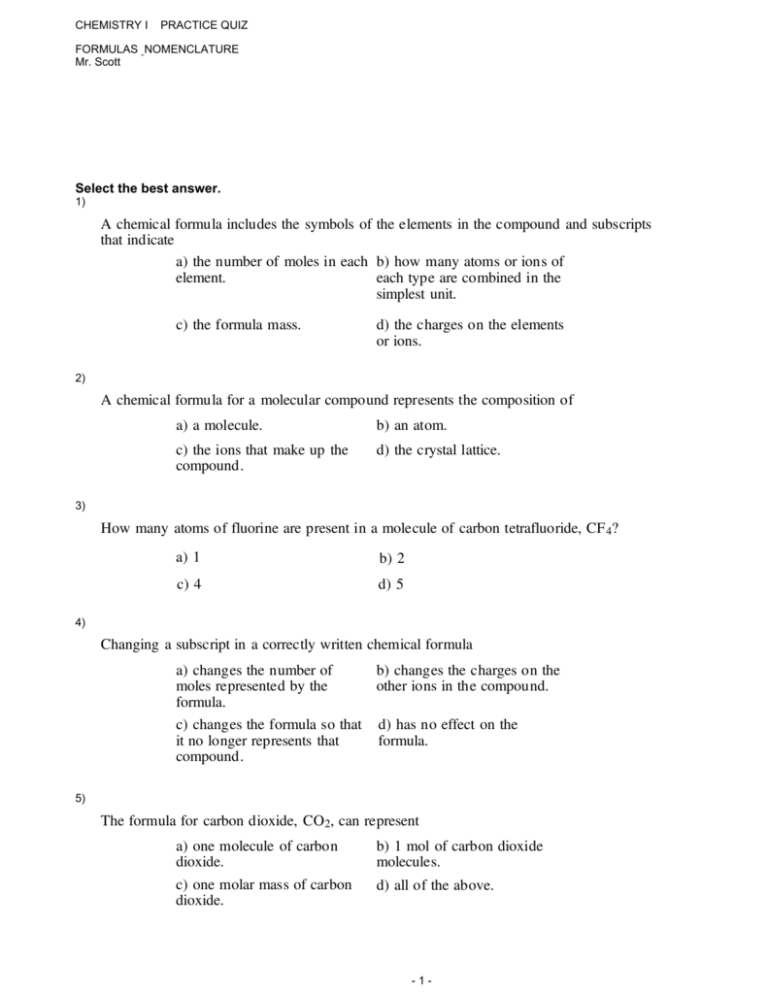
Name the compound n2o2 using the stock system. A chemical formula includes the symbols of the elements in the compound and subscripts that indicate A chemical formula for a molecular compound represents the composition of How many atoms of fluorine are present in a molecule of carbon tetrafluoride CF4. How many atoms of flourine are present in a molecule of carbon tetraflouride CF4. SF6 is the only stable molecule among the two.
What is the oxidation number of carbon in H2CO3aq please explain i dont understand why it gave that whole. Tap again to see term. Oxygen is almost always -2.
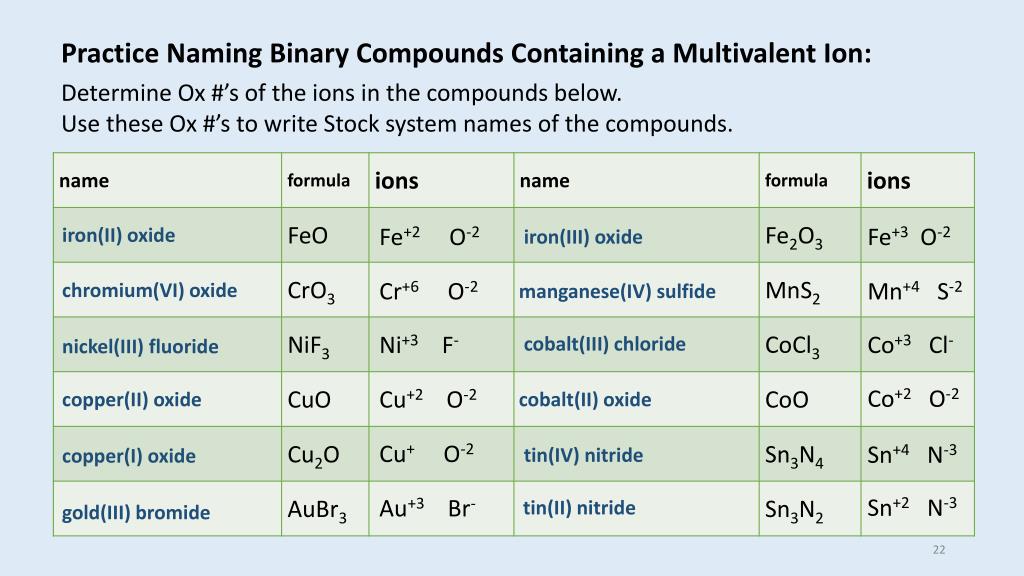
Oxidation Number And Chemical Formula Chemistry. NCl3 nitrogenIII chloride Determine the oxidation numbers for iron oxide. A chemical formula includes the symbols of the elements in the compound and subscripts that indicate.
A chemical formula for a molecular compound represents the composition of. There can also be several of each element such as Fe 2 O 3 or SnBr 4. In the electrolysis of fused molten calcium chloride the product at the anode is a Cl-1 b Cl2 c Ca2 d Ca.
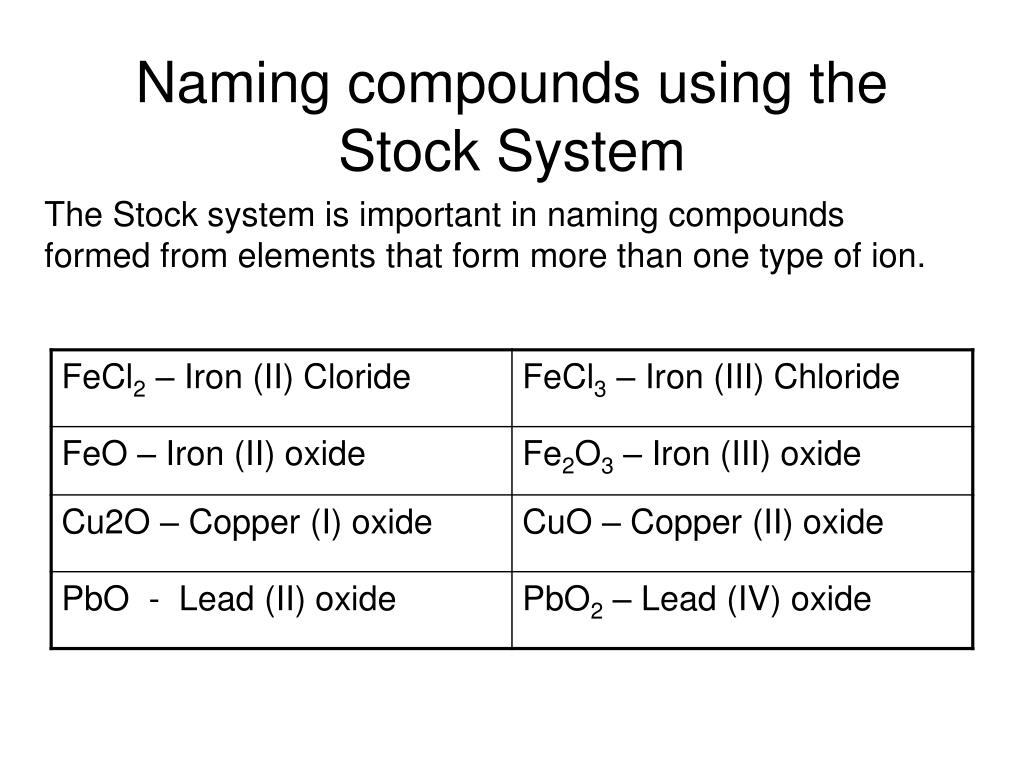
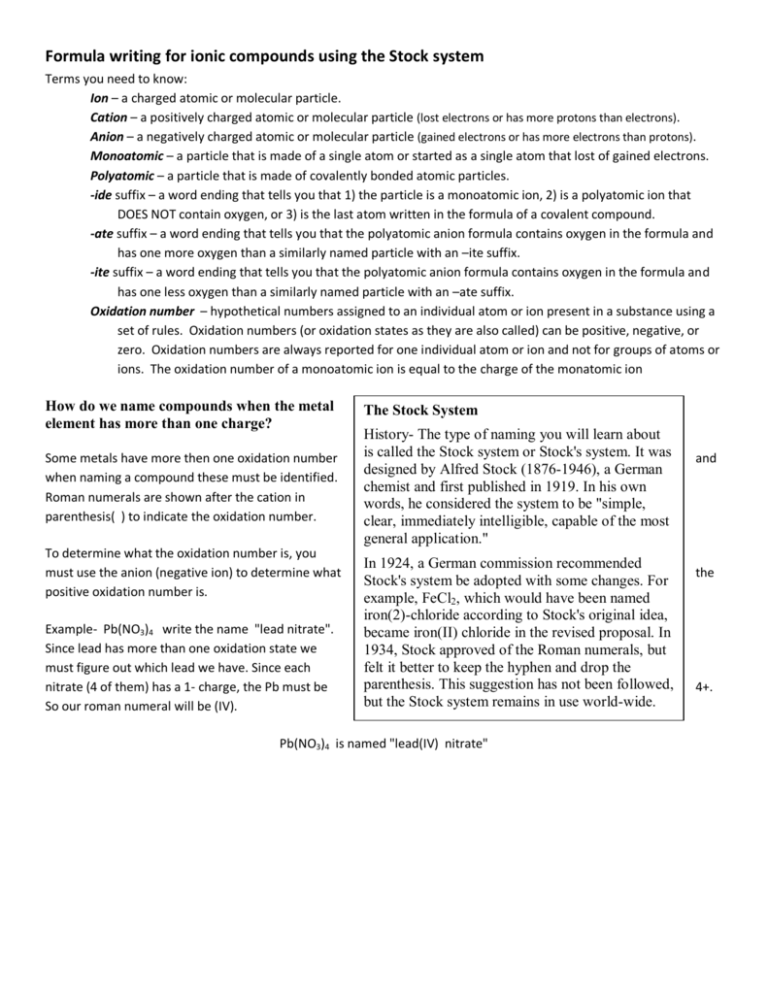
Name the compound N2O2 using the Stock system. 0 This number is reduced relative to that one. Name the compound SO2 using the Stock system sulfurIV oxide.
Commission Free Global Stocks Trading With Industry Leading Spreads on PC Mobile. Number of atoms or ions of each element that are combined in the compound. 1 test answers.
- Answers Carbon monoxide. Calculating The Oxidation State Of Carbon Name the compound N2O2 using the Stock system.
Oxygen is almost always -2.
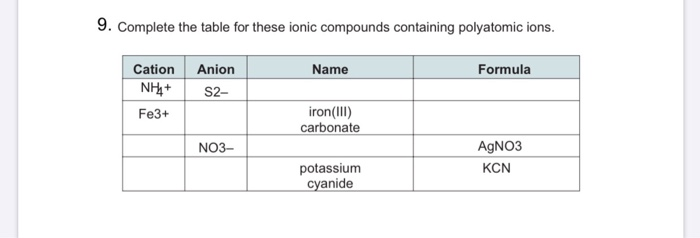
Its common name is Mercuric oxide. So if you are asked what the oxidation number of oxygen is in CO2 the correct answer is -2. Oxygen is almost always -2. Tap card to see definition. Covalent bond 251 Carbon monoxide 245 Chemical compound 56 Molecule 44 Ionic bonding 36 Chemical polarity 33 Ionic compound 33 Cobalt 26 Oxygen 21 Carbonyl group 19 Nonmetal 16 Ammonium 16 Ammonia 15 Carbon 14 Metal 12 Electronegativity 12 Chemical element 11 Chemical bond. Name the following covalent compound CO. Number of atoms or ions of each element that are combined in the compound. A chemical formula includes the symbols of the elements in the compound and subscripts that indicate A chemical formula for a molecular compound represents the composition of How many atoms of fluorine are present in a molecule of carbon tetrafluoride CF4. It is not necessary to indicate the number of cations and anions in the compound because it is understood that the total positive charges carried by the cations must equal the total negative charges carried by the anions.
Calculating The Oxidation State Of Carbon Name the compound N2O2 using the Stock system. Name the compound N2O2 using the Stock system. Name the following covalent compound CO. Oxygen is almost always -2. So if you are asked what the oxidation number of oxygen is in CO2 the correct answer is -2. Covalent bond 251 Carbon monoxide 245 Chemical compound 56 Molecule 44 Ionic bonding 36 Chemical polarity 33 Ionic compound 33 Cobalt 26 Oxygen 21 Carbonyl group 19 Nonmetal 16 Ammonium 16 Ammonia 15 Carbon 14 Metal 12 Electronegativity 12 Chemical element 11 Chemical bond. In CO2 the oxidation number for oxygen is -2.


































Post a Comment for "Name The Compound N2o2 Using The Stock System"